
pyrite is also known as "fools gold"
Source: James St. John, Pyrite crystal-lined vug in vuggy, sucrosic dolostone

pyrite is also known as "fools gold"
Source: James St. John, Pyrite crystal-lined vug in vuggy, sucrosic dolostone
Pyrite is a mineral composed of two elements, iron and sulfur. One atom of iron combines with two atoms of sulfur to create iron sulfide (FeS2). Crystallization can create many forms, with simple cubes resembling the typical crystals in a salt shaker being particularly common. Mineral collectors prize the octahedron crystals found at Barger's quarry in Rockbridge County near Lexington.1
Because of its appearance and presence in metamorphic/volcanic rocks which can contain gold, pyrite is known as "fool's gold."
Sulfur is dissolved as continents erode, then transported by rivers to the oceans. Until about 1 billion-550 million years ago, oxygen levels were low in the deep oceans. In that reducing environment, sulfur combined with dissolved iron and pyrite crystals formed in seafloor sediments.
Then the chemistry of the atmosphere changed during what is called the Neoproterozoic Oxygenation Event or the Second Great Oxidation Event, increasing oxygen levels significantly in the deep oceans. Since that time, oxygen levels in the atmosphere and the oceans have risen to present levels.
Most sulfur atoms transported even into the deep ocean now combine with four oxygen atoms and are deposited as sulphates rather than pyrite. Pyrite can still forms on land where oxygen levels are low, such as in swamps and where groundwater creates a reducing rather than oxidizing environment.
The global increase in oxygen that altered the chemistry of sulfur crossed an ecological threshold. The extra oxygen made it possible for animals to thrive, in addition to cyanobacteria that previously survived when the atmosphere had low oxygen levels. Predation by animals triggered the Cambrian "explosion" of life starting around 540 million years ago. Soft-bodied animals such as the ancestor of trilobites took advantage of the extra oxygen in the water to create shells rich in calcium and phosphate, leading to limestone deposition on the ocean floor.2
Pyrite has been mined in Virginia to obtain both iron and sulfur. To extract the sulfur, pyrite ore was heated to produce sulfur dioxide. That gas was bubbled through water to create sulfuric acid. In addition to its use in manufacturing and to produce gunpowder, sulfuric acid was used to refine oil to produce kerosene.
As the United States experienced the Industrial Revolution, sulfur was needed for manufacturing of glass, soap, bleach, textiles, paper, dye, medicine, sugar, rubber, and fertilizer. Between 1916-1917 as World War I raged in Europe and demand for gunpowder skyrocketed, the price per ton for sulfur tripled. In 1917, Virginia was the largest producer of pyrite in the United States and supplied over 1/3 of the total mined that year. After the demand sank following the end of World War I, the price dropped and pyrite mines closed.3
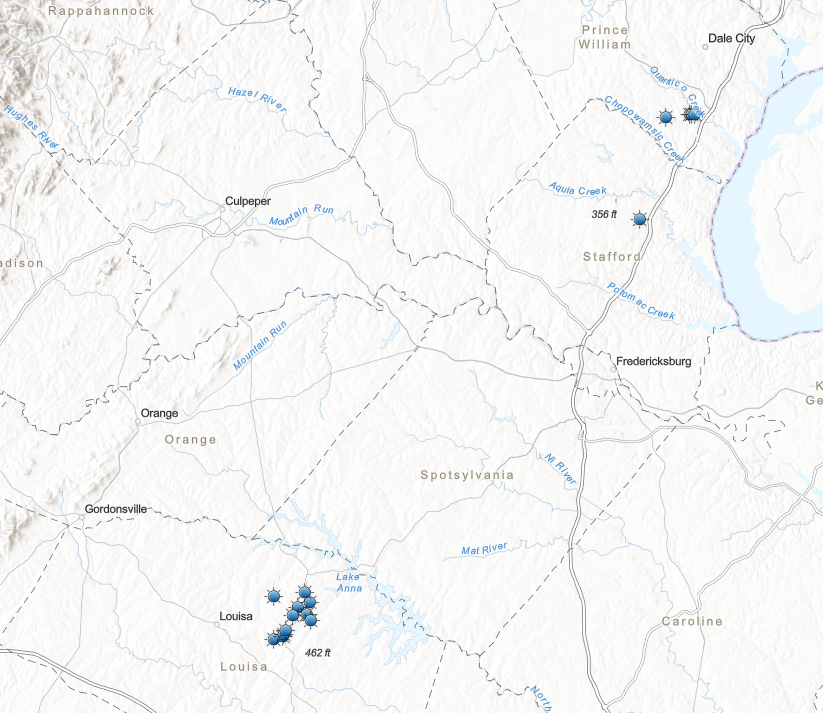
most pyrite mines in Virginia were in northern Louisa County
Source: Laura Caswell, Gold-Pyrite Belt of Virginia
Louisa County was a major pyrite producer, with at least 19 sulfur-pyrite mines. Other major pyrite mines in Virginia included the Cabin Branch Mine on Quantico Creek in Prince William County and the Austin Mine in Stafford County, near modern Aquia Harbor. The ore body at the Austin Mine was only five feet wide. A shaft was dug 646 feet deep to obtain sufficient ore for processing between the mine opening in 1906 and closing in 1920.4
There is a gold-pyrite belt in the Piedmont physiographic province. The gold and pyrite are part of the Chopawamsic Formation, formed from continental crust as volcanic and sedimentary rocks were metamorphosed around 470 million years ago. The pyrite ore was emplaced when the Chopawamsic Terrane was created or when it was accreted to the edge of "Virginia" over 400 million years ago, or perhaps during the Alleghenian Orogeny 250 million years ago when Pangea was formed.
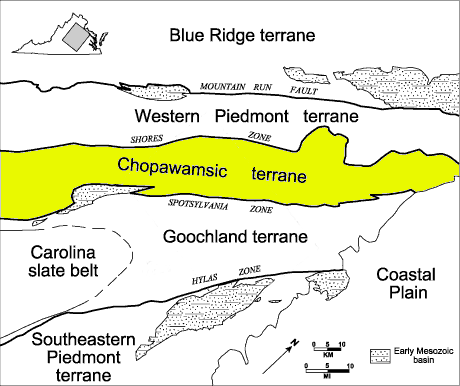
pyrite mines in the Piedmont are concentrated in a gold-pyrite belt on the western edge of the Chopawamsic Terrane
Source: US Geological Survey (USGS), Geology of the National Capital Region - Circular 1264, The Goochland-Chopawamsic Terrane Boundary, Central Virginia Piedmont (Figure 1)
In the Ordovician Period, the Chopawamsic Terrane was an island arc or chunk of continental crust off the shoreline of what became Virginia. Based on analysis of the age of zircons, the terrane is peri-Gondwanan rather than Laurentian. That means the crustal material in the Chopawamsic Terrane was derived from the same source as the crust of the Africa continent, not North America.
The Chopawamsic Terrane was transported by tectonic movement and accreted to the coastline of Virginia, expanding what is now the North American tectonic plate. The collision of the island arc and the coastline triggered the Taconic Orogeny, which pushed up 20,000' high mountains and pressed continental crust down just as deep. Heat and pressure at depth produced hydrothermal fluids into which minerals dissolved. The fluids transported iron, sulfur, gold, and silica (quartz) through fractures in the bedrock. The fluids, when cooled, created zones and veins of solid mineral ores.5
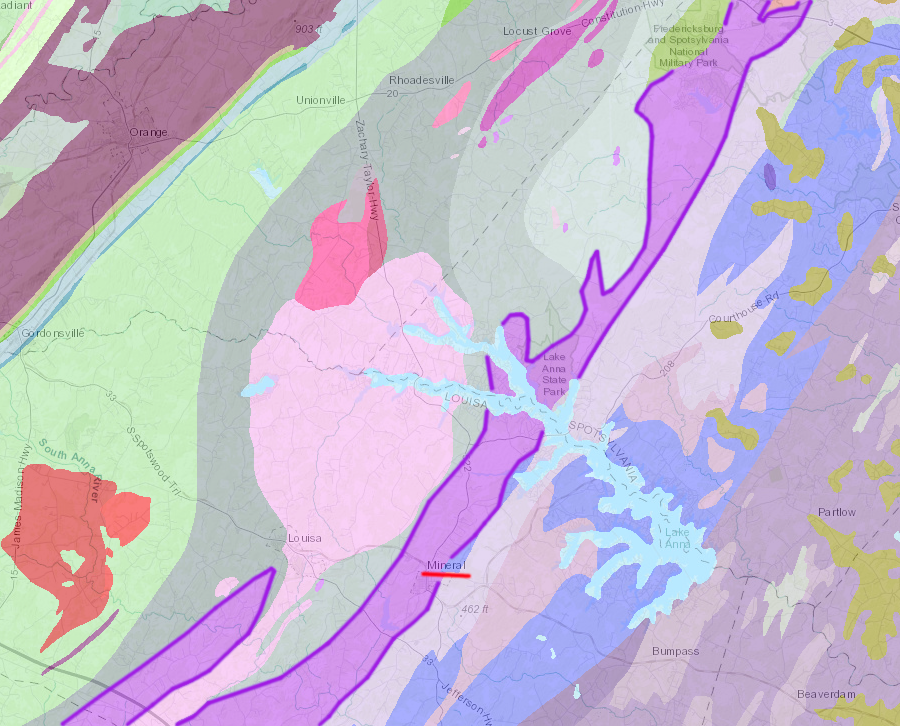
pyrite was mined from the Chopawamsic Formation (purple) north of Mineral in Louisa County
Source: Virginia Department of Mines, Minerals and Energy, 1993 Geologic Map of Virginia
The Sulphur Mine in Louisa County was discovered in 1834. It was opened originally to obtain iron from the Chopawamsic Formation. The iron ore near the surface was a highly oxidized gossan, an ore rich in iron after other minerals were removed by weathering and leaching. The lens body was about 50' thick.
The gossan was excavated and smelted at the Rough and Ready and Victoria furnaces into pig iron. Mining stopped briefly in 1874 when the gossan deposit was exhausted and the miners reached harder bedrock enriched with pyrite. That bedrock was still rich in sulfur and required blasting to excavate.
The mine was re-opened in 1881, after dynamite was available rather than just black powder. When the mine reopened, industrial development had increased the demand for sulfur. As the name implies, the Sulphur Mine was reopened to obtain primarily sulfur rather than iron. One shaft was extended over 700' deep to harder bedrock. Mining ceased around 1920, when the price for sulfur dropped after World War I ended and cheaper sources of sulfur were developed.
Reclamation of the Sulphur Mine occurred in the 1970's and 1980's, followed by a renewed effort in 2010-2011. Open shafts were closed and tailings piles were leveled. After adding limestone, soil was placed on top of the tailings and trees planted.
Pyrite and gold mining in Louisa County has left Contrary Creek as a severely damaged stream. Excavations at the Arminius, Boyd Smith, Sulphur, and Allah Cooper mines exposed sulfur-rich bedrock. Pyrite is a mineral from which the sulfur easily combines with oxygen to create sulfates. There are no pyrite crystals on Virginia Beach; they all oxidized and dissolved into sulphates during transport towards the Atlantic Ocean.
Rainwater reaching sulfur-rich rocks on Contrary Creek spurs the chemical reaction in which pyrite dissolves into iron hydroxide and sulfuric acid long after mining has ceased. The sulfuric acid dissolves lead, manganese, iron and zinc in the mining tailings and bedrock.
The groundwater emerging into Contrary Creek results in high concentrations of heavy metals and sulphates, and pH levels at 2.7. That is too low (too acidic) to support life. The acid drainage from the sulfur-rich rock damages Contrary Creek significantly, but is diluted once it reaches Lake Anna.6
In Carroll County, the "Gossan Lead" was mined for copper and iron prior to the Civil War. After World War II, the Gossan Lead produced pyrite for manufacturing sulfuric acid. The ore was shipped to Pulaski for roasting to extract the sulfur. Iron was a valued byproduct, shipped by rail to the steel mills in Birmingham, Alabama.
The Gossan Lead deposit consisted of a series of elongated lenses; mineralization may have occurred in multiple phases. The sulfides were emplaced in a brecciated zone, likely when thrust faults moved and hot water flowed more readily.
Sulfides near the surface were altered into sulfates, carbonates, or other soluble compounds Rain and groundwater transported the soluble compounds deeper into the weathering bedrock, where they were precipitated to create an enriched mineralized zone. Above that zone, the "gossan" layer was enriched with iron oxides.
The US Geological Survey examined the Gossan Lead after World War II in a search for possible remaining copper deposits. The Bureau of Mines drilled 25 holes, with over 9,000' of exploration borings. The Federal agencies found a gossan layer as much as 30' thick. Below it:7
Particularly within the Chopawamsic Terrane, it is common to encounter acid sulfate soils on top of marine sediments. As described in Stafford County's Soils Testing Policy:8

in 1934, acid from tailings maintained a barren landscape in front of the mine superintendent's house at the Cabin Branch Mine
Source: National Park Service, Unsightly and Abandoned
Most Virginia soils have a pH between 4.5-5.5. The top layers of soils may overly minerals that generate acid byproducts (such as sulfuric acid) when they are exposed to oxygen and weather, but over thousands of years the oxidation is complete near the surface. If the highly-acid byproducts have dissolved in groundwater and eventually washed away, plant roots can grow well in the A and B horizons of soil. The oxidation state of weathered soil is revealed by its orange and yellow color.
However, a road, stormwater pond, or other construction project may expose deeper sulfide-rich minerals below the water table that still remain in a reduced state. That condition is revealed by the gray, steel blue, or black color of the soil. The darker color also is a major clue for delineating wetland soils in a reduced state.
Once exposed to oxygen, chemical reactions occur and acidic byproducts are formed. Acid soils typically have a pH below 3.8; that seriously limits which plant species can grow there. Around Stafford Regional Airport, some soil was so acidic that the pH was 1.8. Calcium-rich sidewalks were stained orange with iron oxides/hydroxides, and the Airport Authority worried that its concrete materials would lose strength over time as cement dissolved.
Techniques for stabilizing the pH at a higher level and supporting the growth of vegetation included adding lime-stabilized biosolids and organic matter. Application of biosolids was done in 2002 and again in 2014 at Stafford Regional Airport. The biosolids came from the District of Columbia Blue Plains treatment facility and the Washington Suburban Sanitation Commission (WSSC) Piscataway treatment plant.9
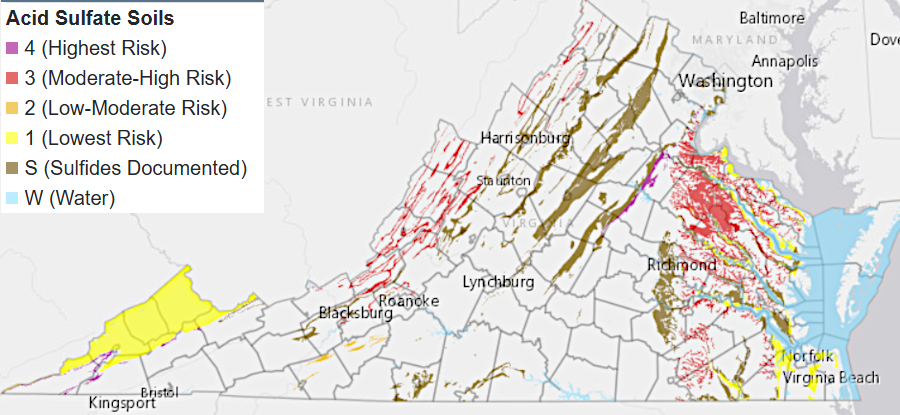
acid sulfate soils form on top of sulfur-rich formations
Source: Virginia Tech, VALEN - Virginia's Land and Enery Navigator

the trail to the old pyrite mine in Prince William Forest Park shows black gravel from Triassic Period basalt covering the Cretaceous Period sedimentary formation underneath